More information on Vector Production
Reagents
The facility will stock many packaging systems available for lentiviral- and oncoretroviral vectors. These reagents can be made available to investigators provided that they have permission from the original source to use them. An effort will be made to stock the most up-to-date packaging systems to be able to provide investigators with state-of-the-art advice and reagents for their gene transfer efforts.
Oncoretroviral Vector Production/Transfection of constructs into packaging cells
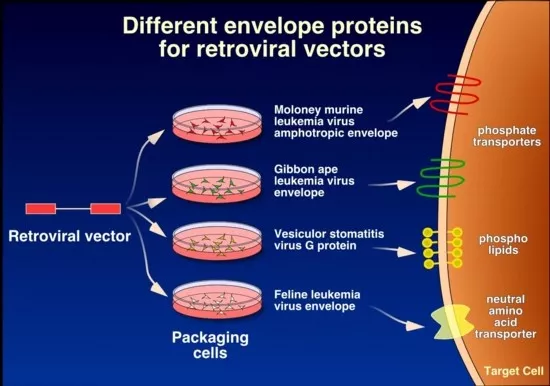
For this service the customer will have to provide a small amount (100ug) of high purity vector plasmid and a restriction enzyme map. The DNA has to be endotoxin free to ensure successful transfection. For production of permanent oncoretroviral clones, the constructs will be transfected into a primary packaging cell (often Phoenix eco, ampho or GP) and then the primary vector supernatant will be used to transduce the secondary and final packaging cells (ecotropic into amphotropic and vice versa). If deemed necessary in order to obtain a high enough titer, clones will be generated from the polyclonal producer cell line and 10-20 clones will be titered to obtain a high titer producer clone. The following vector types with different tropism defined by their respective envelope protein are available: Ecotropic, Amphotropic, gibbon ape leukemia virus (GALV) and vesicular stomatitis virus-G (VSV-G). Since it is possible to generate permanent packaging producer clones for all of these, the customer can upscale production himself by standard tissue culture techniques and when desired, concentrate the vectors even further. VSV-G packaged vectors can be concentrated by ultracentrifugation, others are limited to filtration techniques with the possibility of 10-fold concentration. Protocols for concentration can be provided on request. If the customer is merely interested in transient oncoretroviral production (for example from Phoenix cells), we will provide this service.
Oncoretroviral Vector Titering
To characterize the vector packaging cells and their product, the vectors will be titered using a selectable marker. The enhanced green fluorescent protein (eGFP) gene or its variants in other colours are the most common markers used to titer viruses. Tthe assays are done by FACS. Vectors will be titered on 3T3 cells, HeLa cells or HT1080 cells depending on the envelope used and the wishes of the customer. Alternatively, the neomycin resistance gene is can be used. The neomycin resistance gene is selected for by G418, a neomycin analogue. We strongly advise beginners in the viral vector field to include a selectable marker gene in their vectors together with the gene of interest. This makes it a lot easier to titer the vectors and determine gene transfer efficiency of their target cells of interest. The choice of selectable marker gene depends on the experiment. Therefore, the customers are encouraged to contact the Vector unit before the construct is made to optimize the utility of the vector for the customer. Titering vectors that lack a selectable marker will not be performed.
Lentiviral vector production
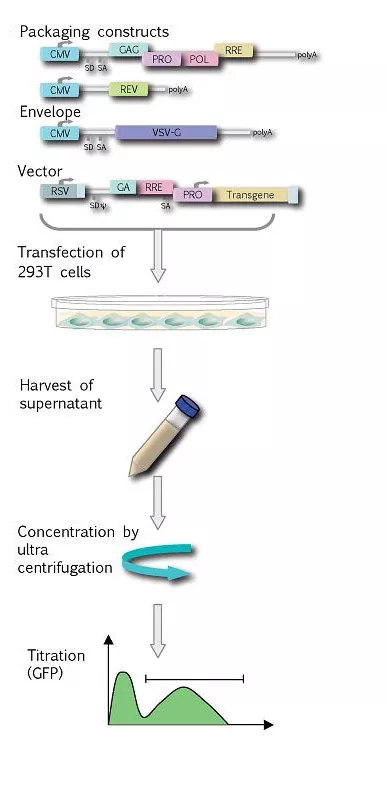
Lentiviral vectors based on HIV-1 will be produced transiently by transfection into 293-T cells using the 4-plasmid system containing the vector, gag-pol, rev and VSV-G genes on separate plasmids. This system cannot produce wt HIV due to the lack of the envelope genes and the accessory protein genes. The vector supernatant is harvested from the transfected cells on day 2 and 3. Following this, the vector can be concentrated approximately 100 fold by ultracentrifugation.
Design Ann CM Brun, Dep of Molecular Medicine and Gene Therapy, Lund
Lentiviral vector titering
Lentiviral vectors will be titered on HeLa cells or 293 cells depending on the choice of the customer. As with oncoretroviral vectors, it is recommended to include a selectable marker gene in the vector together with the gene of interest. This will be used for the titration as described above for oncoretroviruses.
The Vector unit does not test the presence of wild-type HIV in the lentiviral vector preparations. In contrast, the HIV-1 based system, lacks many of the HIV genes as explained above. Wild type HIV-1 can therefore not be made by homologous recombination of the various plasmids in the packaging system used. It is thus theoretically not possible to generate wild type HIV from the preparations and it would incur more danger and potential contamination risks to do testing for HIV in the Vector unit.
Quality Control
The minimum quality control for all oncoretroviral vector clones and lentiviral vectors will be titering. This guarantees the transducibility of the vector and presence of functional marker gene.