Parkinson's Disease is a neurodegenerative disorder that affects almost 8 million people worldwide, causing movement difficulties due to the loss of dopamine-producing nerve cells in the brain. Although it can also affect younger individuals, it mainly occurs in older people, making it a growing concern as the world's population continues to age. Despite various drug developments over the last 25 years, none have been proven to slow the disease's progression or restore lost circuits.
Since the 1970s, researchers at Lund University have been investigating stem cell-based therapies as a potential regenerative treatment for Parkinson’s Disease.
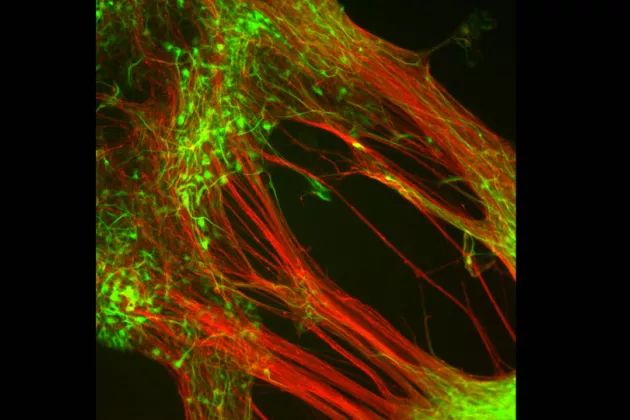
In 2012, after years of experiments in the lab clarified exactly which growth factors and signals stem cells needed to develop into dopamine-producing cells, Professor Malin Parmar, and Associate Professor Agnete Kirkeby, a postdoctoral researcher in the Parmar lab at the time, published a breakthrough method to grow transplantable dopamine cells from embryonic stem cells.
Collaborating within different EU networks, and with neurologists and neurosurgeons from the University of Cambridge and Skåne University Hospital, they have since worked to manufacture these cells for clinical use, resulting in the creation of the STEM-PD product. It involves creating new dopamine-producing nerve cells from stem cells in the lab and transplanting them into the brains of patients with Parkinson’s Disease. The hope is that these new cells will mature, integrate, and function similar to the nerve cells the patients have lost.
The Swedish regulatory authorities approved the STEM-PD product in October 2022, and the first transplant of stem cell-derived nerve cells was administered to a patient in February 2023. “Earlier this year, we initiated the STEM-PD clinical trial with the main aim to investigate the safety of this method but also to assess whether the transplanted dopamine cells from stem cells survive in the patient's brains and can replace the nerve cells and their functions lost in the disease,” says Professor Malin Parmar, who is leading the STEM-PD team.
Associate Professor, Agnete Kirkeby, who led the preclinical development of the product explains: "If the therapy works, we hope to see that the patients with time will reduce their dopaminergic medication and that they will regain some motor function in the absence of medication.”
The team behind STEM-PD has now published a data-packed article detailing all the in vitro and in vivo tests conducted on the product to achieve regulatory approval for transplantation among the first group of patients. "Releasing such data provides an unusual window into the comprehensive and rigorous testing needed to bring novel stem cell therapies into clinical trial. This is highly valuable information for other scientists in the field and may hopefully help to speed up clinical development of stem cell therapies also for other diseases," says Dr. Kirkeby.
If the outcome of this initial academic clinical trial demonstrates the safety of the treatment after 12 months, research partner Novo Nordisk A/S intends to conduct further trials and product development, with the potential of bringing the STEM-PD product to the market.
“We are excited to have reached this milestone in Parkinson's Disease research, this is an important step towards making stem cell therapies available as a safe and effective treatment for large patient groups,” says Malin Parmar.