Services
Full-Service iPS Generation
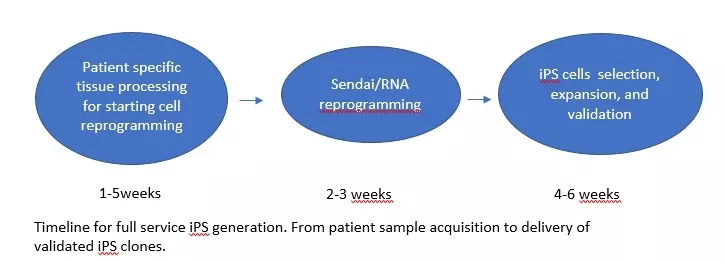
Full-service generation of novel iPS lines can be ordered. The ethical approval of collecting patient samples for reprogramming is the responsibility of the users laboratory and a copy must be provided to the core. The starting cell material from patients/donors may be from skin biopsies, peripheral blood, cord blood, etc. We currently use the Sendai reprogramming system that is based on a non-integrating viral system. Currently under development is an RNA based reprogramming method that will be available shortly.
The Service will include:
- processing starting cell material
- reprogramming Sendai/RNAtra
- manually picking 12 clones
- we guarantee at least 2 validated iPS clones (3 frozen vials each)
- validation includes pluripotency marker test (PCR or Immuno)
User access to Cell Culture Clean Rooms
The human Pluripotent Stem Cell Core provides the facility and infrastructure for maintenance and expansion of human ES and iPS cell lines. The facility is complete with advanced and state-of-the-art pluripotent stem cell tissue culture equipment, reagents, cell storage facilities all maintained by the core facility staff. Users of the facility must undergo a 1 hour introduction training prior to working in the facility. LAF hoods are booked via online calendar. Users may use the facility in a limited manner. The work in the core is limited to ES and iPS cell culture, maintenance, and expansion; not differentiation of the pluripotent stem cell lines. The price to use the facility is based on a per hour hood charge and user reagent consumption.
Provide human ES cell lines
We have banked numerous Harvard and WiCell generated human embryonic stem cell lines and have agreements in place to distribute these lines to users via an accelerated MTA approval contract at significantly reduced cost to local users.
Access to control iPS lines
The iPS lines generated in the core may be obtainable to other users depending on the ethical approval agreements under which they were established. We provide a list of iPS lines produced at the facility and act as the intermediate for users to be able to establish contact with those who have the lines. We also help provide advice on how to write the ethical agreements to facilitate a broader use of novel iPS lines in research collaborations.
Consultation and Advanced Cell Culture Techniques
Starting a new project? We provide consultation service to help you get started and design the project correctly. We also provide training for advance culture techniques. We also have a library of protocols that may be accessed by users (free).
Karyoptyping of pluripotent stem cell lines
This service is in-part performed at another accredited laboratory. Either the user or the core can process the samples, and the core will deliver the samples. The results will be passed on the user. The amount of time required for processing by the core staff will determine the price.
Routine Mycoplasma testing
This service is provided on a monthly basis free of charge for all samples currently in culture in the facility. Additional testing of samples is able to be performed at a surcharge.
Protocol database
We currently have over 20 protocols available to users detailing pluripotent stem cell techniques, such as splitting, freezing, EB formation etc. This service is provided free of charge. For complete list contact us.
CONTACT
Niels-Bjarne Woods
Director (ES/iPS)
Phone: +46 46 222 37 62
niels-bjarne [dot] woods [at] med [dot] lu [dot] se (niels-bjarne[dot]woods[at]med[dot]lu[dot]se)
Henrik Ahlenius
Director (ES/iPS)
Phone: +46 46 222 42 59
Henrik [dot] Ahlenius [at] med [dot] lu [dot] se (Henrik[dot]Ahlenius[at]med[dot]lu[dot]se)
Qianren Jin
Manager
Phone: +46 46 222 77 51
Qianren [dot] Jin [at] med [dot] lu [dot] se (Qianren[dot]Jin[at]med[dot]lu[dot]se)
Emanuela Monni
Manager
Phone: +46 46 222 05 15
Emanuela [dot] Monni [at] med [dot] lu [dot] se (Emanuela[dot]Monni[at]med[dot]lu[dot]se)