Cell and Gene Therapy Core - Services & Charges
The services provided by the Cell and Gene Therapy Core Facility are available to all internal and external academic customers. StemTherapy and MultiPark users have priority access.
We offer the following services:
Vector Production Services (AAV & Lentiviral)
Please note that new prices are in place as of 01 January, 2024. International customers please cellandgenetherapy [at] med [dot] lu [dot] se (contact us) for pricing.
Consultation and Training
At the Cell and Gene Therapy Core Facility, we offer training, consultation, design and trouble-shooting in all core related areas.
The first meeting is always free. further consultation, training and designs are charged at 1000SEK/h.
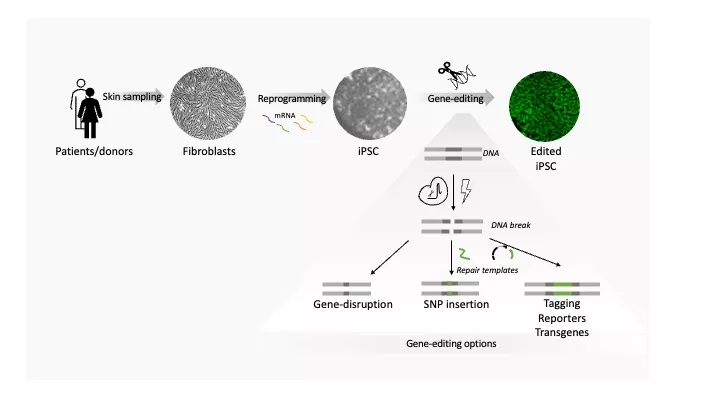
Fibroblast derivation (9 000SEK)
- Derivation, expansion, and freezing of fibroblasts from freshly collected skin biopsy.
- Cell authentication (STR) and molecular karyotyping included
- Delivery of mycoplasma tested fibroblasts, at least 300k/vial x 3 vials.
mRNA-based reprogramming from human fibroblast cells (72 000 SEK)
- Reprogramming is done with feeder free system.
- Picking at least 10 colonies
- Deliver 2 clonally expanded mycoplasma free iPSC lines (15 x 300k cells).
- Quality controls as follows:
- Cell authentication (STR), pluripotency and undifferentiated state confirmation, molecular karyotype, g-banding and iPS growth pattern and morphology analysis.
Sendai based reprogramming from human fibroblast cells/blood (75 000SEK)
- Reprogramming is done with feeder free system
- Picking at least 12 colonies
- Expansion of top three to four colonies
- Deliver 2 clonally expanded mycoplasma free iPSC lines at around (15 x 300k cells) with quality controls as follows:
- Sendai virus clearance, cell authentication (STR), pluripotency and undifferentiated state confirmation, molecular karyotype, g-banding and iPS growth pattern and morphology analysis.
Edits can be performed in researcher- or core-provided iPS-lines (starts at 65 000SEK)
- We offer:
- KO edits
- Point mutation correction/insertions
- Reporter and tagged lines
- Design of the gRNAs and repair templates are included.
- Edits will be performed using RNP nucleofection.
- Expected delivery of 2 correctly edited clones, (300k/vial x 5 vials)
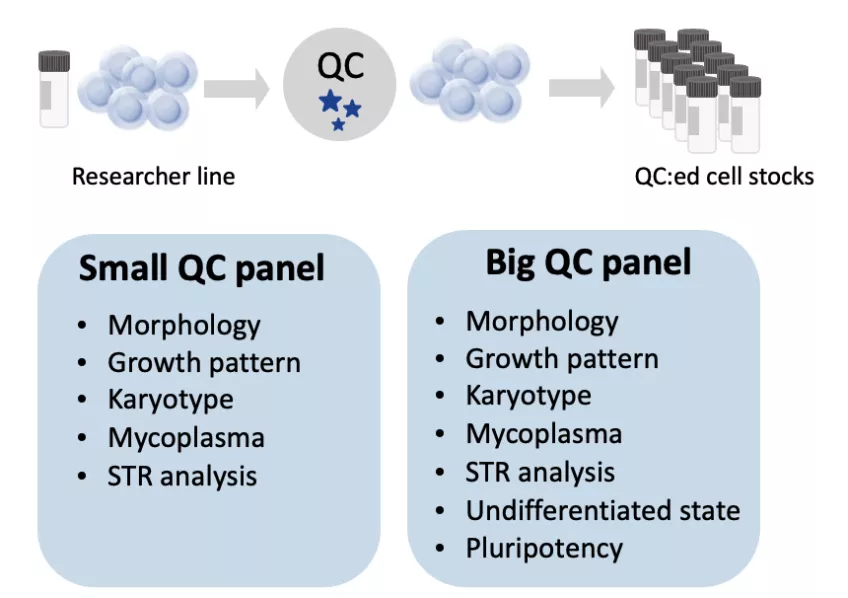
- QC included: basic characterization, including cell authentication (STR), undifferentiated state (FACS) and molecular karyotyping.
- Optional deliveries (price per clone)
- Extra cell stocks (24 or 48 extra vials)
- Additional edited or unedited clones
- Optional QC of
- g-banded karyotyping
- Pluripotency test (tri-lineage differentiation)
- Top 5 off-target analysis
- Cell stocks 20x300k cells, small QC panel
- Cell stocks 48x300k cells, small QC panel
- Cell stocks 20x300k cells, large QC panel
- Cell stocks 48x300k cells, large QC panel
The facility is complete with advanced and state-of-the-art pluripotent stem cell tissue culture equipment, reagents and cell storage facilities all maintained by the core facility staff. Users of the clean rooms must undergo a 1 hour introduction training prior access. LAF hoods are booked via an online calendar. Users may use the facility in a limited and availability dependent manner. The work in the core is limited to ES and iPS cell culture, maintenance, and expansion; not differentiation of the pluripotent stem cell lines.
Hood booking: 300SEK/hour | Reagents: List price | Introduction: 500SEK
Our Pricing Strategy
The CAGT core is a not-for-profit academic entity, our fees include the cost of consumables and a per hour salary cost. We are partly subsided by StemTherapy and Multipark in terms of rent and salaries.
We have increased prices for 2024 due to the increase in inflation and prices for consumables, decreased subsidy from StemTherapy and through re-calculation of cost of services (which until now have been much underestimated).
However, the fees we charge for services are still heavily subsidized as users only pay for consumables and hours that are actually used. The fees for each service are displayed alongside the service description on this page.
mRNA Production Services
The Cell and Gene Therapy Core Facility has begun to offer mRNA production services, including:
| Validated mCherry or GFP mRNA; aliquots of 1,5 pmol (1,5 ul) | 3000 SEK |
| Custom mRNA production | 15000 SEK (plus plasmid cost) |
CRISPR Services
CRISPR services at the Cell and Gene Therapy Core Facility include the following:
- CRISPR edits in induced pluripotent stem cells (iPSCs) (see above)
- Experimental design (in a multitude of cell types and species)
- gRNA design (CRISPR-cut, CRISPRi, CRISPRa)
- HDR design and strategies for tagging and correction
- Cloning protocols for gRNA insertion
- Access to off-the shelf plasmid backbones (15ug)
- Cut and edit validations strategies
Vector Production Services
Full service production of lentivirus and AAV
The core provides investigators access to state-of-the-art vector technology for preclinical studies and other basic research applications to be able to provide users with highly reproducible, high-titer vector preparations.
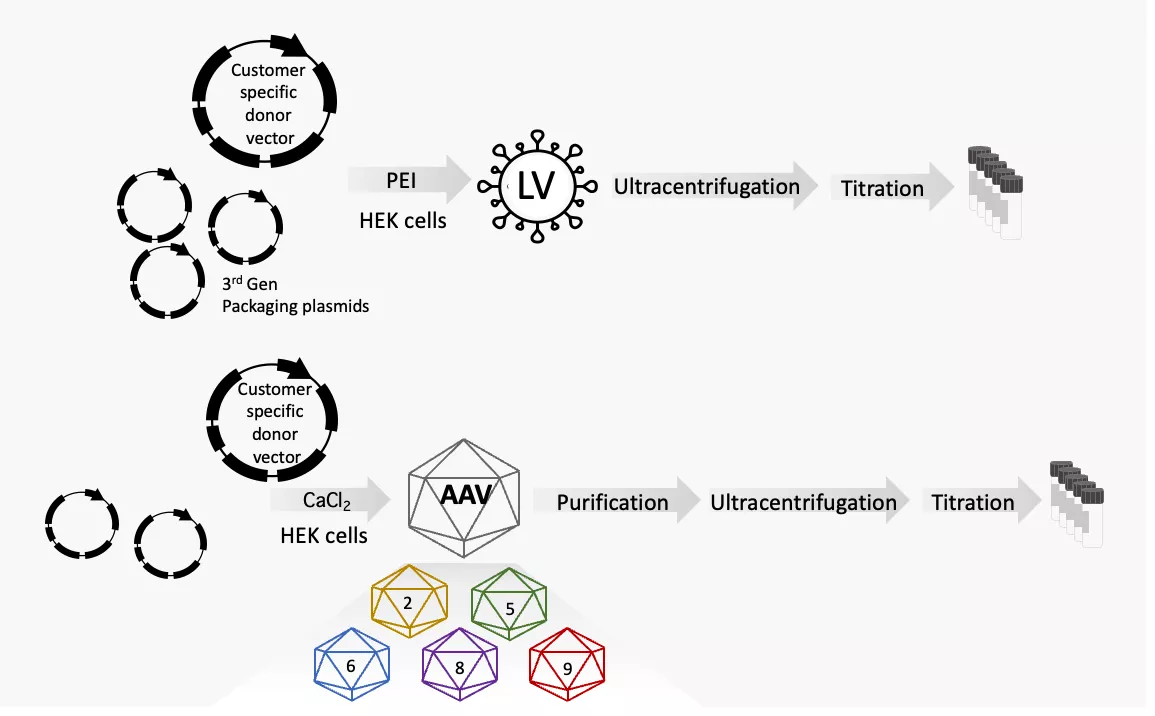
How we produce the vector preps:
Transfection of the packaging cell with the user provided transfer vector along with core provided packaging constructs, followed by ultracentrifugation/concentration of the supernatants. The concentrated supernatants are titrated via FACS or qPCR.
Lentiviral vectors are offered in 3 sizes:
**Please note new names of our sizes***
- small (1 plate): delivers around 90ul of 107-108 TUI/ml*, 3000 SEK
- medium (3 plates): delivers around 300ul of 107-108 TUI/ml*, 6000 SEK
- large (10 plates): delivers around 1000ul of 107-108 TUI/ml*, 15000 SEK
*depending on plasmid size and titration protocol
Useful Information:
- For Lentiviral vectors please provide the following amounts of donor plasmid DNA: LV-small 20ug, LV-medium 55ug, LV-large 160ug.
- The quality of the DNA matters, please ensure that you have performed restriction digest and/or sequencing and have an OD ratio of 1.7-2.0 and deliver at a concentration of 0.5-1.0ug/ul.
cellandgenetherapy [at] med [dot] lu [dot] se (Contact us for vector related services)
The AAV services provided by the Cell and Gene Therapy Core core include:
- AAV based vector production (200ul, approx. 1·1013, 12000SEK per batch)
- Storage and handling of core-produced AAV vectors according to agreement.
- Robust quality control and titration of vectors
- A wide variety of AAV serotypes (AAV 2, 5, 6, 8, 9)
Useful Information:
- For AAV vectors provide 100ug of the transfer vector plasmid, deliver at a concentration of 0.5-1.0ug/ul.
- Please ensure that you have performed digestion with SmaI to verify the ITR region.
cellandgenetherapy [at] med [dot] lu [dot] se (Contact us for vector related services)